Beginning in 2015, NCQA began enhancing the HEDIS® measurement set to focus on a new reporting framework for measures derived from Electronic Clinical Data Systems (ECDS).
MY2022 reporting adds three ECDS measures
Prenatal Immunization Status was the first ECDS measure to be publicly reported in measurement year 2020. Three additional measures will be publicly reported in measurement year 2022:
- Adult Immunization Status (AIS-E)
- Postpartum Depression Screening and Follow-up (PDS-E)
- Prenatal Depression Screening and Follow-up (PND-E)
The Adult Immunization Status measure assesses the percentage of adults that are up to date for influenza, Td/Tdap, zoster, and pneumococcal vaccines.[1] In the HEDIS 2023 public comment, NCQA indicated that some modest changes to the age groups and pneumococcal vaccines may be forthcoming.[2] This was echoed in CMS’ 2023 call letter that referenced updated guidelines for pneumococcal vaccines from the Advisory Committee on Immunization Practices[3].
NCQA’s assessment of ECDS data submissions was encouraging for public reporting of AIS-E. While claims are still the predominant source of immunization data, the percentage of immunizations sourced from EHR and immunization registry sources increased by more than 50% in measurement year 2020 compared to measurement year 2019.
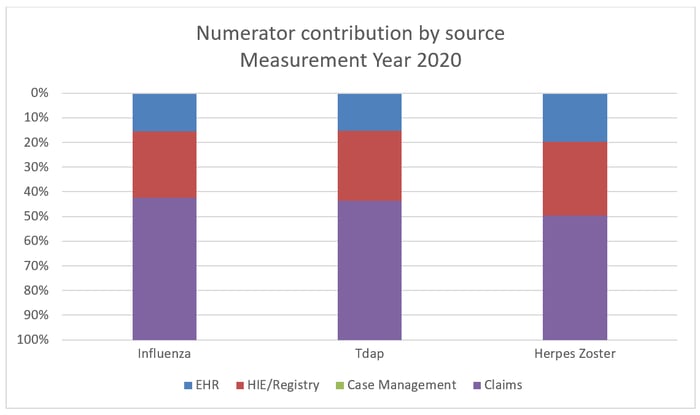
Rates of influenza and pneumococcal vaccination increased significantly for the Medicare product line—from 18.4% to 32.6% and from 15.7% to 26.2%, respectively. It will be important for health plans to assess the completeness of data capture of vaccinations and pursue additional sources of vaccination data such as local immunization registries.
With public reporting of AIS-E, NCQA also proposes to streamline reporting and retire three immunization measures currently reported with the Consumer Assessment of Healthcare Providers and Systems (CAHPS®) health plan survey method in measurement year 2023:
- Flu Vaccinations for Adults Ages 18–64 (FVA)
- Flu Vaccinations for Adults Ages 65 and Older (FVO)
- Pneumococcal Vaccination Status for Older Adults (PNU)
MY2023 defines four additional behavioral health measures to ECDS reporting
For measurement year 2022, NCQA introduced Postpartum Depression Screening and Follow-up (PDS-E) and Prenatal Depression Screening and Follow-up (PND-E) as two initial measures in the area of behavioral health.
Looking ahead to measurement year 2023, NCQA has published a schedule[4] indicating four additional measures that will be publicly reported, all of which continue the MY2022 trend with a behavioral health focus:
- Depression Remission or Response for Adolescents and Adults (DRR-E)
- Depression Screening and Follow-Up for Adolescents and Adults (DSF-E)
- Unhealthy Alcohol Use Screening and Follow-Up (ASF-E)
- Utilization of the PHQ-9 to Monitor Depression Symptoms for Adolescents and Adults (DMS-E)
ECDS measures related to behavioral health present new challenges to health plans. These measures rely heavily on clinical data to assess that a screening was performed. The definition of screening often relies on multiple screening instruments with different scoring methods and thresholds. Also, screening data is not transactional and is dependent on both the presence of a standard code and a score value, for which there really is no administrative equivalent.
Additionally, many state jurisdictions have implemented privacy laws specific to protecting recipients of mental health treatment. In these jurisdictions, providers have expressed concern that sensitive mental health information is protected and should not be routinely exchanged with health plans.
NCQA is aware of these issues and has stated “challenges persist particularly regarding standardized data capture of behavioral health information at the point of care and efficient sharing of relevant information between health care systems.”[5] The challenge is evident when analyzing the relatively small percentage of plans that were able to submit a rate greater than 0% for these measures for measurement year 2020.
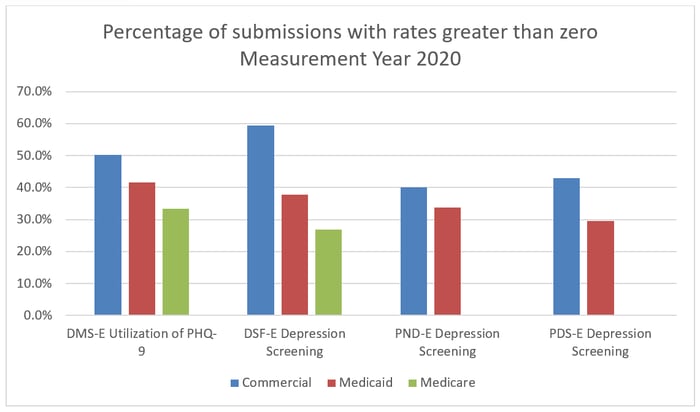
Solving the clinical data challenge
There is a significant investment required to develop a sustainable legal and technical framework for the acquisition of comprehensive clinical data for performance reporting. However, health plans can solve this data governance challenge by working with their provider network to enable access to this unique clinical data.
Why is the clinical data conundrum so complex? There are multiple reasons, none of which cannot be solved, but all of which will require operational and technological expertise. First, clinical data is structured very differently than transactional data. Plus, the FHIR standards are not very mature and there is significant variation in the implementation across data sources. This means that new skills need to be introduced into your HEDIS team to support data validation.
An additional challenge is the lack of a national patient identifier,[6] so, health plan members are identified with multiple patient identifiers across their servicing providers. Patient matching algorithms must be applied to associate data in the medical record with the patient enrolled in the health plan. While simple matching based on name and date of birth may work for the majority of records presented, ongoing monitoring for data integrity is required. A small percentage of records may need further intervention to solve for patient identity issues. Examples below are indicative of challenges to patient matching based on the name:
|
Member information in enrollment |
Patient information in EMR |
|
Smith III, William |
Smith, William III |
|
Allen, Patricia |
Allen, Patty |
|
Scott, Rebecca Jane |
Scott, Jane |
|
Lowe, Charles E |
Lowe, Charles |
|
Stewart, Andrew |
Stewart, AJ |
|
Anderson, Jill (married name) |
Jones, Jill (maiden name) |
|
Barnes-Campbell, Mary |
Campbell, Mary Barnes |
|
St Lawrence, John |
St. Lawrence, John |
|
De Souza, Paul |
DeSouza, Paul |
|
DAngelo, Noah |
D’Angelo, Noah |
|
Muniz, Christine |
Muñiz, Christine |
As new electronic clinical data systems become available to your health plan, be sure to plan for the necessary audit steps, such as primary source validation. Ensure that you are correctly categorizing data for both supplemental data usage in traditional measures and with the source system of record (SSOR) for usage in ECDS measures.
Reference our Summary of Measure Changes for MY2022 for details on highlighted measures and corresponding implications for your health plan. With more than 20 years of experience helping plans with technology to support the collection of clinical data, we provide expert guidance for evolving HEDIS requirements. Contact us today to see how we can help you.
[1] https://www.ncqa.org/hedis/measures/adult-immunization-status/
[2] https://www.ncqa.org/blog/hedis-public-comment-period-is-now-open/
[3] https://www.cms.gov/files/document/2023-announcement.pdf
[4] https://www.ncqa.org/hedis/the-future-of-hedis/hedis-electronic-clinical-data-system-ecds-reporting/
[5] https://www.ncqa.org/wp-content/uploads/2021/11/Special-Report-Reporting-Results-for-Measures-Leveraging-Electronic-Clinical-Data-for-HEDIS.pdf
[6] https://www.ahima.org/news-publications/press-room-press-releases/2022-press-releases/patient-id-now-coalition-disappointed-congress-continues-to-include-rider-to-stifle-progress-on-patient-identification/
Written by Amy Salls
Sr. Director, Revenue and Quality Analytics






